AKLIEF Cream: AN INNOVATIVE APPROACH TO
ACNE CARE.
AKLIEF Cream is the first retinoid cream with
proven results in treating facial and truncal
acne vulgaris.1
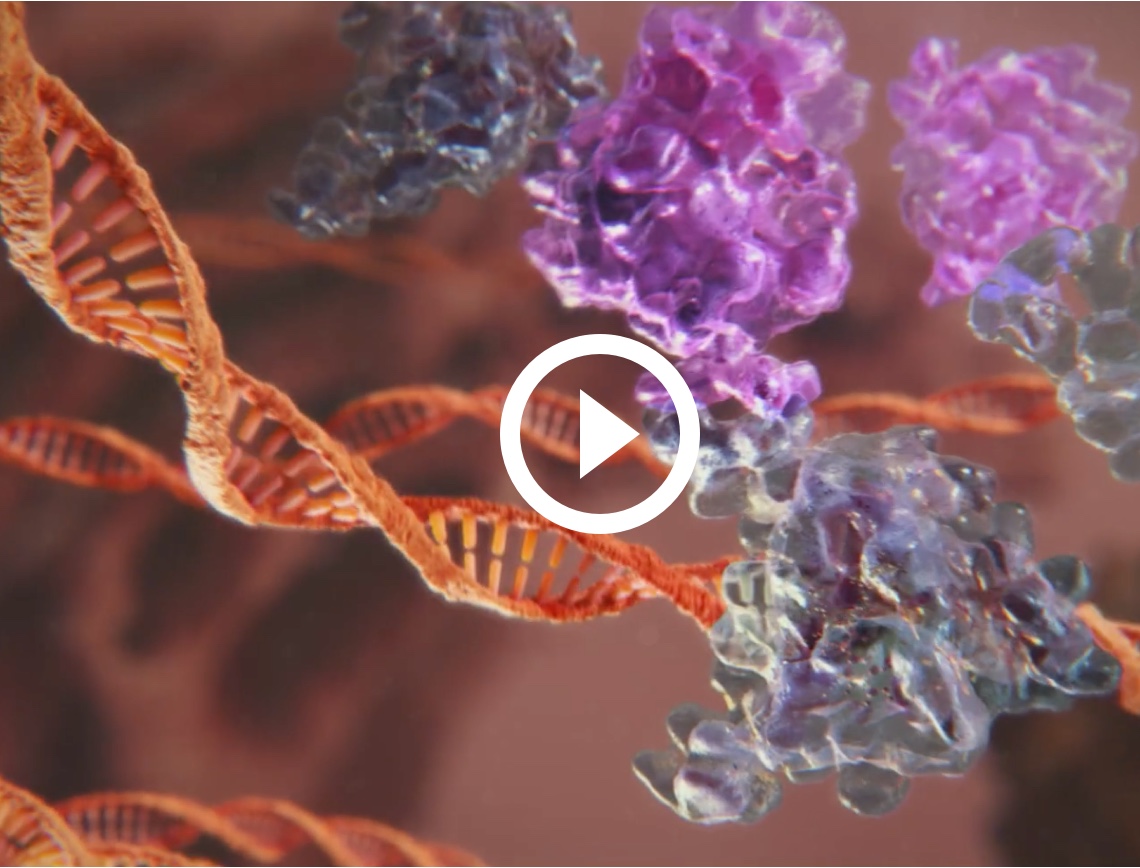
THE IMPORTANCE OF RAR-γ IN THE TREATMENT OF ACNE VULGARIS.
In a study of human skin biopsies, the percentage of RAR found in the skin was2:

RAR-α (12-14%) and RAR-β (not detected) were significantly less prevalent.2
Targeting RAR-γ with retinoids for acne has several important functions in acne via gene modification2
- Inflammation modulated via AP-12
- Epidermal turnover and normalizing cell growth2
- Keratinization
- Desquamation
A UNIQUE ACNE TREATMENT DELIVERY SYSTEM.
Simulgel® 600 PHA6
- Non-greasy
- Allows for homogenous dispersion of active ingredients
- Not sensitive to pH changes

2 emollients6-8
Cyclomethicone and medium-chain triglycerides allow for greater spreadability

Allantoin6,8
A known skin-conditioning agent and protectant

Paraben-free6
Emollients are mostly lipids and oils which hydrate and improve skin softness, flexibility, and smoothness.11
PHA=(P)olysorbate 80, Iso(H)exadecane, and copolymer of (A)crylamide.
AKLIEF Cream in action THE POWER OF PRECISION.
The first topical retinoid to specifically target RAR-γ, the most prevalent RAR in the skin.2,11
PROVEN EFFICACY IN TREATING
ACNE VULGARIS IN
2,000+
PATIENTS ENROLLED IN
2 CLINICAL TRIALS11
RAPID RESULTS.* POWERFUL
EFFICACY. EXCELLENT TOLERABILITY.11
*Indicates a 2-week time frame on both the face and the trunk.11

ACNE THAT'S BEYOND WHAT'S IN PLAIN SIGHT.
Patients with truncal acne may not freely talk about it with their doctors for various reasons. However, if left untreated, it can have a negative impact on their daily lives.6,10