Facial acne is only half the story.
Patients with truncal acne often suffer from self-consciousness and social withdrawal.2 They also feel embarrassed by the acne on their trunk, but still focus more on their facial acne when speaking with their dermatology providers.1,2
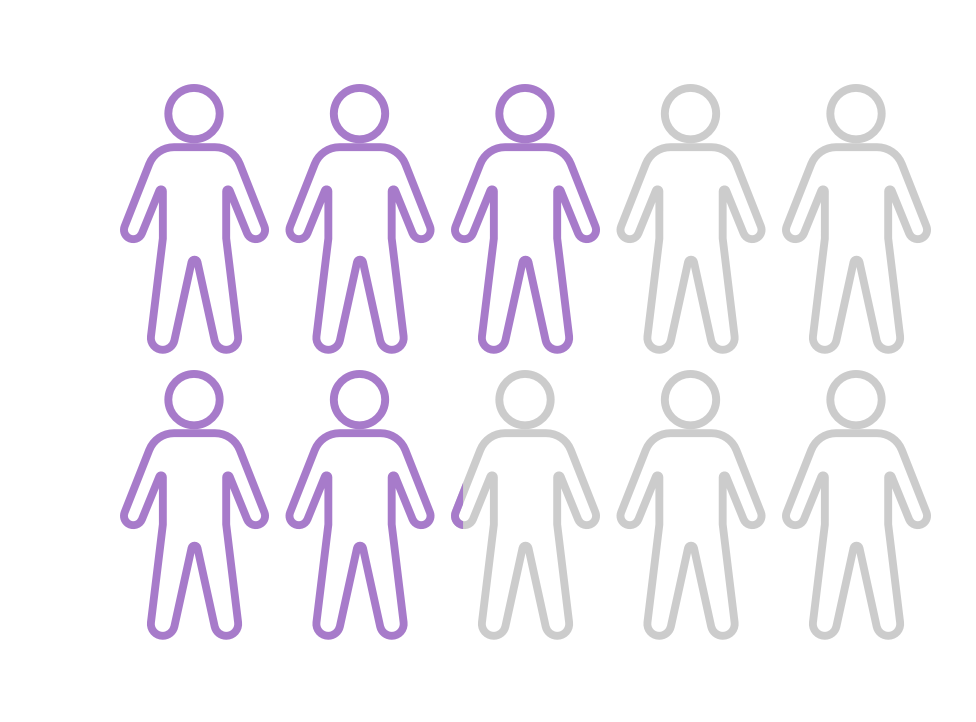
MOST patients want treatment FOR TRUNCAL ACNE.1